Global Initiative for Asthma: Asthma management and prevention, 2019
Global Initiative for Asthma (GINA), 2019
Global Initiative for Asthma (GINA), 2019
The 2019 GINA strategy report represents the most important change in asthma management in 30 years. GINA no longer recommends treatment with short-acting beta2-agonists (SABA) alone in the light of mounting evidence that SABA-only does not protect patients from severe exacerbations and that regular or frequent use of SABAs increases the risk of exacerbations. GINA now recommends that all adults and adolescents with asthma should receive either symptom-driven or daily low dose inhaled corticosteroid (ICS)-containing controller treatment to reduce the risk of serious asthma attacks.
BOX 1. WHAT IS KNOWN ABOUT ASTHMA?Asthma is a heterogenous disease, usually characterised by chronic airway inflammation. Its two defining features are:– A history of respiratory symptoms such as wheeze, shortness of breath, chest tightness and cough that vary over time and in intensity, AND– Variable expiratory airflow limitationAsthma exacerbations can be fatal. They are more common and more severe when asthma is uncontrolled, or in some high risk patients but can occur even in people taking asthma treatment, so all patients should have an asthma action planAsthma treatment should be customised to the individual patient, taking into account their symptom control, risk factors for exacerbations, phenotype characteristics and preferences, as well as the safety, efficacy and cost of medication
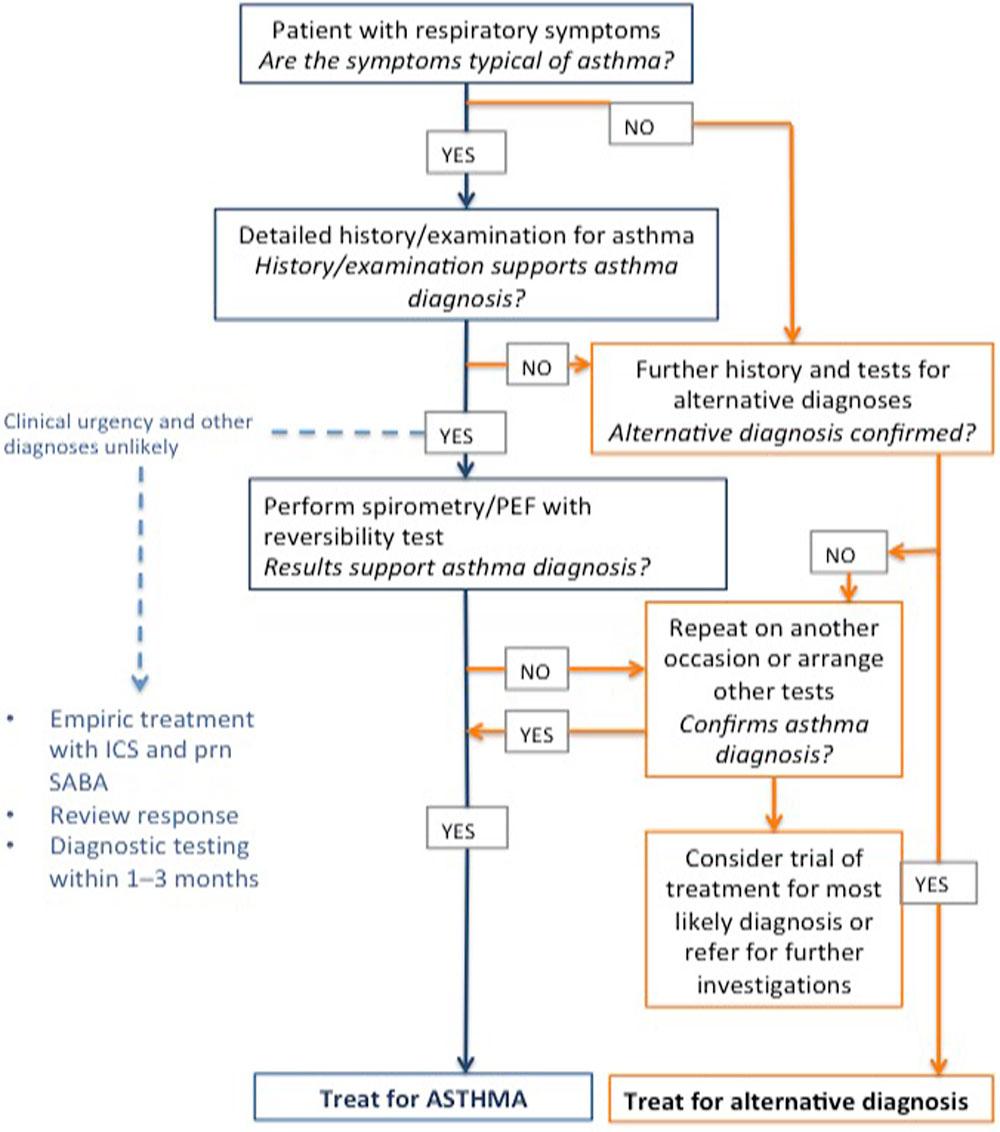
CRITERIA FOR MAKING A DIAGNOSIS OF ASTHMA
1. History of variable respiratory symptoms such as wheeze, shortness of breath, chest tightness, cough
People with asthma generally have more than one of these symptoms
Symptoms:
- Vary over time and in intensity
- Are often triggered by exercise, laughter, allergens or cold air
- Often occur with or become worse with viral infections
2. Evidence of variable expiratory airflow limitation
FEV1/FVC ratio is normally >0.75-0.80 in adults and >0.85 in children. Excess variability occurs if:
- FEV1 increases by more than 200ml and >12% of baseline value (>12% predicted in children) after inhaling bronchodilator (bronchodilator reversibility)
- Average daily diurnal peak expiratory flow (PEF) variability is >10% (>13% in children)
- FEV1 increases by >12% and 200ml from baseline (>12% of predicted in children) after 4 weeks of anti-inflammatory treatment (excluding respiratory infections
The greater the variation, or the more times excess variation is seen, the more confident you can be in the diagnosis of asthma
For 25-35% of patients with a diagnosis of asthma in primary care, the diagnosis cannot be confirmed. If the basis for the diagnosis has not been documented, confirm with objective testing, e.g. spirometry.
ASSESSING A PATIENT WITH ASTHMA
Take every opportunity to assess patients with asthma, particularly when they are symptomatic or after a recent exacerbation, but also when they ask for a prescription refill. Routine review should be scheduled at least once a year.
- Assess symptom control over the last 4 weeks (Box 2)
- Identify any modifiable risk factors for poor outcomes, such as ICS not prescribed; poor adherence; incorrect inhaler technique; high SABA use (>1x200 dose inhaler per month)
- Measure lung function before starting treatment, 3-6 months later then at least once a year in most patients.
- Identify any comorbidities, including rhinitis, chronic rhinosinusitis, gastroeosophageal reflux (GORD), obesity, obstructive sleep apnoea, depression and anxiety, which may contribute to respiratory symptoms, exacerbations and poor quality of life, and which may complicate asthma management
- Record the patient’s treatment and ask about side effects
- Watch the patient using their inhaler to check their technique
- Have an open, empathetic discussion about adherence
- Check that the patient has a written asthma action plan
- Ask the patient about their attitudes and goals for their asthma
BOX 2. LEVEL OF ASTHMA SYMPTOM CONTROLIn the past 4 weeks has the patient had:Daytime symptoms more than twice a week? Yes/NoAny night time waking due to asthma? Yes/NoReliever needed more than twice a week? Yes/NoAny activity limitation due to asthma? Yes/NoNone of these = Well controlled1-2 of these = Partly controlled3-4 of these = Uncontrolled
Most patients can achieve good asthma control with regular controller treatment, but some do not, and further investigation is needed.
Watch the patient use their inhaler. Compare with a device-specific checklist and correct errors. Recheck frequently. Discuss adherence and barriers to use.
Confirm the diagnosis of asthma. If lung function is normal during symptoms, consider halving ICS dose and repeating lung function assessment after 2-3 weeks.
Check for (and remove) potential risk factors such as smoking, beta-blockers, NSAIDs, allergen exposure.
Consider stepping up treatment
Refer to a specialist or severe asthma clinic if asthma is still uncontrolled after 3-6 months on Step 4 treatment, or earlier if symptoms are severe or there are doubts about diagnosis.
MANAGEMENT OF ASTHMA
The long term goals of asthma management are risk reduction and symptom control. The aim is to reduce the burden to the patient, and to reduce their risk of asthma-related death, exacerbations, airway damage and medication side effects.
Asthma management involves a continuous cycle of assessment, treatment adjustment and response review.
Major change in GINA 2019 recommendations for mild asthma
For safety, GINA no longer recommends starting treatment for asthma with SABA only. GINA recommends that all adults and adolescents with asthma should receive ICS-containing controller treatment, to reduce their risk of serious exacerbations and to control symptoms.
ICS controller options include:
- As needed, low dose ICS-formoterol (Off-label; evidence only with budesonide-formoterol), or if not available, low dose ICS taken whenever SABA is taken (Off-label, combination or separate inhalers)
- Regular ICS or ICS-LABA every day, plus as-needed SABA, OR
Maintenance and reliever treatment with ICS-formoterol
The new recommendations aim to:
- Reduce the risk of asthma-related exacerbations and death, including in patients with so-called mild asthma
- To provide consistent messaging about the aims of treatment, including the prevention of exacerbations, across the spectrum of severity
- To avoid establishing a pattern of patient reliance on SABA early in the course of the disease
Patients with apparently mild asthma are at risk of serious adverse events, and 30-37% of adults with acute asthma, 16% of patients with near-fatal asthma and 15-20% had symptoms less than once a week in the previous three months.
Inhaled SABA has been first-line treatment for asthma for 50 years, dating from a time when asthma was thought to be a disease of bronchoconstriction. However, airway inflammation is found in most patients with asthma, even in those with intermittent or infrequent symptoms.
Patient satisfaction with, and reliance on SABA treatment is reinforced by its rapid relief of symptoms. Patients often do no see the need for additional treatment.
Higher use of SABA is associated with adverse clinical outcomes. Over-use of SABA (defined as more than3 inhalers per year [average 1.7 puffs a day]) is associated with an increased risk of severe exacerbations, and using ≥12 inhalers a year is associated with a higher risk of death.
STARTING TREATMENT
Initiate ICS-containing treatment as soon as possible after diagnosis is made, because:
- Patients with even mild asthma can have severe exacerbations
- Low dose ICS markedly reduces asthma hospitalisations and death
- Low dose ICS is very effective in preventing severe exacerbations, reducing symptoms, improving lung function, and preventing exercise-induced bronchoconstriction, even in patients with mild asthma
- Early treatment with low dose ICS leads to better lung function than if symptoms have been present for more tan 2-4 years
Consider starting at a higher step (e.g. medium/high dose ICS or low-dose ICS/LABA if the patient has troublesome asthma symptoms on most days, or is waking from asthma once a week or more often.
Consider stepping down after asthma has been well-controlled for 3 months. However, in adults and adolescents, ICS should not be completely stopped.
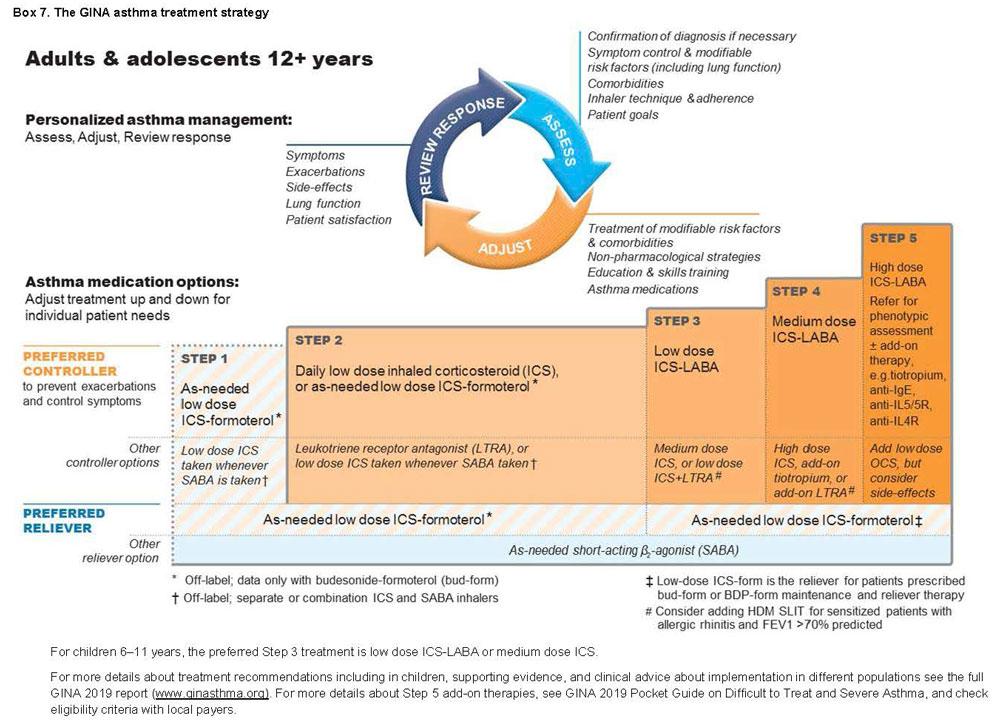
Step 1
For patients with symptoms less than twice a month and no exacerbation factors.
Preferred controller: as-needed low-dose ICS-formoterol (off-label) The evidence for this approach is with low-dose budesonide-formoterol, but BDP-formoterol may also be suitable,
OR
Low dose ICS taken whenever SABA is taken (off-label)
GINA has suggested daily low dose ICS since 2014, but patients with symptoms less than twice a month are unlikely to take ICS regularly, leaving them exposed to the risks of SABA-only treatment, so is no longer recommended.
Step 2
Preferred controllers: daily low dose ICS with as needed SABA
Adding daily ICS has been shown to halve the incidence of severe exacerbations, even in patients with symptoms 0-1 days a week.
Consider adherence before prescribing daily ICS.
OR
As-needed low-dose ICS-formoterol (off-label)
This approach has been shown to reduce severe exacerbations by 64% compared with SABA-only treatment. The most important considerations are to prevent severe exacerbations and to avoid the need for daily ICS for patients with mild asthma.
OR
Low dose ICS taken whenever SABA is taken, either in combination or separate inhalers (off-label). This has been shown to result in similar rates of, or fewer, exacerbations compared with daily ICS.
Daily low-dose ICS-LABA as initial therapy leads to faster improvement in symptoms and FEV1 than ICS alone, but is more costly and the exacerbation rate is similar.
Leuokotriene receptor antagoinists (LTRA) are less effective than regular ICS, particularly for preventing exacerbations.
Step 3
Preferred controller: Low dose ICE-LABA maintenance plus as-needed SABA, OR low dose ICS-formoterol maintenance and reliever therapy
This recommendation is unchanged from 2018.
Step 4
Preferred controller: Preferred controller: Low dose ICE-formoterol maintenance and reliever therapy OR medium dose ICS-LABA maintenance plus as-needed SABA
Other controller options include:
Add-on tiotropium by mist inhaler for patients ≥6 years with a history of exacerbations;
Add-on LTRA
Increasing to high-dose ICS-LABA but consider potential increase in ICS side-effects
Step 5
Refer for phenotypic investigations ± add-on treatment
Patient with uncontrolled symptoms and/or exacerbations despite Step 4 treatment should be assessed for contributory factors, treatment optimised and referred for expert assessment, including severe asthma phenotype, and potential add-on treatment. The GINA Pocket Guide on Difficult to Treat and Severe Asthma v2.0 2019 available at https://ginasthma.org/reports/ provides further guidance for assessment and management.
REVIEWING RESPONSE AND ADJUSTING TREATMENT
Patients should be seen 1-3 months after starting treatment and every 3-12 months after that. Pregnant women with asthma should be reviewed every 4-6 weeks.
After an exacerbation, review within 1 week.
Stepping up
Asthma is a variable condition and controller treatment may need to be adjusted from time to time.
Sustained step up for at least 2-3 months
If symptoms and/or exacerbations persist despite 2-3 months of controller treatment, assess these common issues before stepping up:
- Incorrect inhaler technique
- Poor adherence
- Modifiable risk factors, e.g. smoking
- Whether symptoms are due to comorbid conditions, e.g. allergic rhinitis
Short term step up for 1-2 weeks
By clinician or patient with written asthma action plan, e.g. during viral infection or allergen exposure
Day to day adjustment by patient
For patients prescribed as needed low dose ICS-formoterol for mild asthma, or low dose ICS-formoterol as maintenance and reliever therapy.
Stepping down
Consider stepping down treatment once good asthma control has been achieved and maintained for 3 months to find the lowest treatment that controls both symptoms and exacerbations and minimises side effects.
- Choose an appropriate time to step down (no respiratory infection, patient not travelling, not pregnant)
- Document baseline status (symptom control and lung function), provide a written asthma action plan, monitor closely, and book follow-up visit
- Step down through available formulations to reduce ICS dose by 25-50% at 2-3 month intervals
- Do not completely stop ICS
INHALER SKILLS AND ADHERENCE
Provide skills training
Most patients (80%) cannot use their inhaler correctly, leading to poor symptom control and exacerbations. To ensure effective inhaler use:
- Choose the most appropriate device for the patient before prescribing. Consider medication, physical problems, e.g. arthritis, patient skills, and cost. For ICS by pMDI prescribe a spacer
- Check inhaler technique at every opportunity. Ask the patient to show you how they use the inhaler. Check technique against a device-specific checklist
- Correct using a physical demonstration
- Confirm that you have checklists for each of the inhalers you prescribe, and can demonstrate correct technique
Check adherence
At least 50% of patients do not take controller medications as prescribed, either unintentionally (forgetfulness, cost, misunderstandings) and/or intentionally (not perceiving need for treatment, fear of side effects, cultural issues, cost).
- Ask empathetic questions to identify adherence problems, e.g. ‘How many times a week have you been using your controller inhaler?’, ‘Do you find it easier to remember in the morning or at night?’
- Check medication usage from prescription data
- Ask about attitudes and beliefs about asthma and medication
Interventions that may improve adherence
- Shared decision-making for medication and dose choice
- Inhaler reminders for missed doses
- Comprehensive asthma education (with home visits by asthma nurses)
- Clinicians reviewing feedback about patients’ dispensing records
- Messages when refills are due or overdue
- Directly-observed controller therapy at school (using telemedicine)
WRITTEN ASTHMA ACTION PLANS
All patients should be provided with a written asthma action plan appropriate for their level of asthma control and health literacy so they know how to recognise and respond to worsening asthma.
It should include:
- Usual asthma medications
- When and how to increase medications, and start OCS
- How to access medical care if symptoms fail to respond.
Action plans can be based on symptoms and/or (in adults) PEF. Patients who deteriorate quickly should be advised to seek urgent care immediately.
Medication changes
- Increase frequency of inhaled reliever (SABA or low dose ICS-formoterol)
- Increase controller
- ICS: in adults and adolescents, quadruple dose
- Maintenance ICS-formoterol: quadruple maintenance dose (to maximum formoterol dose of 72mcg/day)
- Maintenance ICS-other LABA: step up to higher dose formulation or consider adding separate ICS inhaler to achieve quadruple ICS dose
- Maintenance and reliever ICS-formoterol: Continue maintenance dose, increase reliever doses as needed (maximum formoterol 72mcg/day)
- Oral corticosteroids (preferably morning dosing; review before stopping)
- Adults: prednisolone 40-50mg, usually for 5–7 days
- For children, 1-2mg/kg/day up to 40mg, usually for 3–5 days
- Tapering not needed if OCS has been given for less than 2 weeks.
MANAGING EXACERBATIONS IN PRIMARY CARE
Assess exacerbation severity while starting SABA and oxygen. Assess dyspnoea (is patient able to speak sentences or only words), respiratory rate, pulse rate, oxygen saturation and lunch function (e.g. PEF). Check for anaphylaxis.
Consider alternative causes of acute breathlessness (e.g. heart failure, upper airway dysfunction, inhaled foreign body or pulmonary embolism).
Arrange immediate transfer to acute care if there are signs of severe exacerbation, or to intensive care if the patient is drowsy, confused or has a silent chest. For these patients, immediately give inhaled SABA, inhaled ipratropium bromide, oxygen and systemic corticosteroids.
Start treatment with repeated doses of SABA (usually by pMDI and spacer), early OCS, and controlled flow oxygen if available. Check response of symptoms and saturation frequently, and measure lung function after 1 hour. Titrate oxygen to maintain saturation of 93-95% in adults and adolescents (94-98% in children 6-12 years).
GINA. Asthma management and prevention, 2019
https://ginasthma.org/wp-content/uploads/2019/04/GINA-2019-main-Pocket-Guide-wms.pdf
Related guidelines
View all Guidelines