Pharmacological management of glycaemic control in people with type 2 diabetes: SIGN, 2017
January 2018
January 2018
This guideline provides evidence-based recommendations and best practice guidance on optimal targets for glucose control for the prevention of microvascular and macrovascular complications, and the risks and benefits of the main therapeutic classes of glucose-lowering agents and insulins currently available for those who require measures beyond diet and exercise to achieve glucose targets.
INTRODUCTION
The immediate purpose of lowering blood glucose in people with type 2 diabetes is to provide relief from symptoms (thirst, polyuria, nocturia, and blurred vision). Thereafter, the aim is to prevent microvascular complications: loss of vision (retinopathy), renal failure (nephropathy), and foot ulceration (neuropathy).
High blood glucose (hyperglycaemia) is also one of the features of diabetes, along with raised blood pressure and cholesterol, which is associated with macrovascular complications (myocardial infarction, stroke, and peripheral arterial disease).
The effects of glucose-lowering therapies on cardiovascular morbidity and mortality are therefore of major importance and not necessarily related to glucose lowering. Until 2010, the majority of clinical trials focused narrowly on glucose control (as assessed by HbA1c [glycated haemoglobin] concentrations), and on the risks of weight gain and hypoglycaemia rather than on cardiovascular morbidity and mortality. Since then, several large cardiovascular outcome trials have been published comparing individual glucose-lowering agents with standard of care.
As a result of the large volume of new evidence that has emerged since the previous guideline (SIGN 116) was published in 2010, including a comparative effectiveness review of diabetes medications by the Agency for Healthcare Research and Quality (AHRQ), SIGN has produced an up to date guideline incorporating new data from cardiovascular outcome trials.
KEY RECOMMENDATIONS
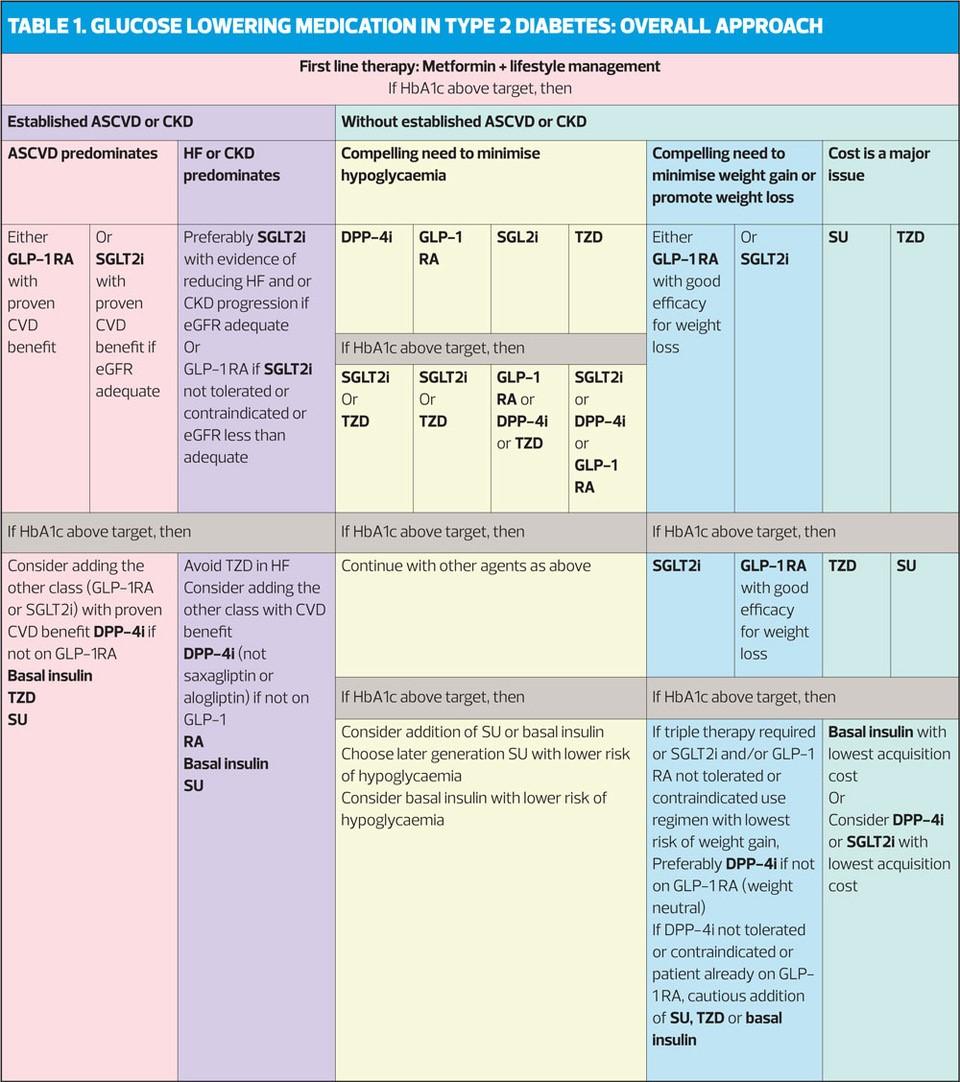
An HbA1c of 53mmol/mol is a reasonable target to reduce the risk of micro- and macrovascular disease. On diagnosis, a lower target of 48mmol/mol may be appropriate. Targets should be set with individuals and should balance benefits with harms, notably hypoglycaemia and weight gain.
First line
Metformin should be considered as the first-line oral treatment option for people with type 2 diabetes.
SGLT2is
In patients with type 2 diabetes and established cardiovascular disease (CVD), sodium glucose co-transporter 2 inhibitors (SGLT2is) with proven cardiovascular benefit (currently empaglifozin and canagliflozin) should be considered.
GLP-1s
Consider glucagon-like peptide-1 (GLP-1) receptor agonists with proven cardiovascular benefit (currently liraglutide) for patients with established CVD.
GLYCAEMIC TARGETS
Reducing HbA1c levels to between 46mmol/mol and 64mmol/mol has been shown to be associated with a reduction in micro- and macrovascular complications in a number of studies, including UKPDS 33, ADVANCE (Action in Diabetes and Vascular disease: Preterax and damicron MR Controlled Evaluation) and ACCORD (Action to Control Cardiovascular Risk in Diabetes). (ACCORD used the range of available therapies to reduce HbA1c rapidly from 67mmol/mol at baseline to a mean of 58mmol/mol, but treatment was stopped early in the intensive therapy group as mortality was significantly higher than in the usual care group.)
A meta-analysis of ACCORD, ADVANCE, UKPDS and VADT (Veterans Affairs Diabetes Trial) showed that intensive glucose control compared with less intensive glucose control resulted in a relative reduction in the risk of nephropathy and retinopathy but no reduction in neuropathy events.
Meta-analyses of the above trials showed that intensive glycaemic control substantial reductions in the risk of major CVD, but did not reduce overall mortality.
Treatment to more intensive glycaemic targets increases the incidence of hypoglycaemia, with significantly more episodes reported in intensive versus conventional therapy groups in most studies.
Patients in intensive control groups, typically treated with metformin, sulfonylureas or insulin, gained more weight than conventional treatment groups in most studies.
SIGN 154 therefore recommends a target of 53mmol/mol to reduce the risk of micro- and macrovascular disease, but a lower target of 48mmol/mol may be appropriate at diagnosis. Targets should balance the benefits with harms, particularly hypoglycaemia and weight gain.
DRUG TREATMENT
Metformin
Metformin is a member of the biguanide class of drugs, and has been used as an effective glucose-lowering agent for around 60 years. Its effects include reduced glucose production in the liver, weight loss or stabilisation, and improved insulin sensitivity.
Despite advances in available treatments, metformin remains a very effective drug. In addition to blood glucose lowering, metformin appears to reduce cardiovascular morbidity and mortality, and is not associated with weight gain or hypoglycaemia. The main adverse effects with metformin are gastrointestinal but these may be reduced by using a modified release formulation.
SIGN 154 therefore recommends that metformin should be considered as the first line oral treatment option for people with T2D. It should be used with caution in patients with renal impairment – use progressive dose reductions with declining kidney function – and avoid in those with severe renal impairment.
Sulfonylureas
Sulfonylureas (SUs) increase endogenous release of insulin from pancreatic β-cells, and they can bring about rapid reductions in blood glucose. For this reason, they are also associated with a higher rate of major hypoglycaemia than many other glucose lowering agents apart from insulin. SUs are also associated with weight gain.
The AHRQ review found no significant differences in effect on HbA1c between SUs and metformin, but there was insufficient evidence to compare SUs with thiazolidinediones (TZDs), DPP4 inhibitors, GLP-1 agonists for long-term effects on HbA1c. A number of trials have compared SUs to newer diabetes treatments but most were designed to show a reduction in adverse effects rather than comparing HbA1c reduction.
SIGN 154 therefore recommends that SUs should be considered as first line oral agents in people for whom metformin is contraindicated or not tolerated, and as second line treatment to other oral therapies. SUs also have a role in triple oral therapy. However, SUs are associated with hypoglycaemia (use with caution in elderly patients) and weight gain.
Thiazolidinediones (TZD)
Pioglitazone is the only TZD still available. It works by increasing insulin sensitivity. It is effective at lowering HbA1c as monotherapy and in dual or triple therapy when combined with metformin, SUs, DPP4 inhibitors or insulin. Some SGLT2 inhibitors, DPP4 inhibitors and GLP-1s are licensed for use with pioglitazone but evidence is lacking for all combinations.
Pioglitazone is associated with weight gain, oedema, increased fracture risk (especially in women) and a small increased risk of bladder cancer. It should not therefore be used in patients with active, or a history of, bladder cancer. Nor should it be used in patients with hepatic impairment or heart failure.
SIGN 154 therefore recommends that pioglitazone should be considered for loweing HbA1c, usually as dual or triple therapy. It should not be used in patients with heart failure. Clinicians should consider the risk of fracture during long-term use of pioglitazone and should make patients aware of the increased risk of peripheral oedema, heart failure, weight gain, bladder cancer and fractures.
DPP4 inhibitors
DPP4 inhibitors are oral agents that block the activity of the enzyme DPP-4, thus prolonging the action of endogenous GLP-1, an incretin hormone which prompts secretion of isulin from pancreatic β-cells and inhibiting glucagon secretion. The five DPP4 inhibitors currently available are alogliptin, linagliptin, saxagliptin, sitagliptin and vildagliptin. DPP4 inhibitors are effective at lowering HbA1c, well-tolerated and associated with lower rates of hypoglycaemia than other agents used in diabetes management.
The AHQR review found that DPP4 provide HbA1c reduction that is:
- In combination with metformin, greater than metformin alone
- Not significantly different to metformin and an SU
- Less than metformin in combination with a TZD, an SGLT2 inhibitor or GLP-1.
DPP4s are ‘weight neutral’ – in trials, DPP4s were associated with smaller weight losses than metformin, SGLT2s and GLP-1s, but greater weight loss than TZDs or SUs. DPP4s can be used as monotherapy for patients for whom both metformin and SUs are contraindicated or not tolerated, or in dual or triple combinations with other agents.
Dose reductions are advised for DPP4s other than linagliptin for patients with moderate or severe renal impairment.
SIGN 154 therefore recommends that DPP4 inhibitors should be considered for lowering HbA1c, usually as dual or triple therapy.
SGLT2 inhibitors
SGLT2 inhibitors work by reducing glucose re-absorption by the kidneys, resulting in increased glucose excretion equivalent to a net loss of 200-300 kcal/day. Their glucose lowering effect is independent of pancreatic β-cell function. The three drugs currently available in this class are canagliflozin, dapagliflozin and empaglifozin.
The AHRQ review found no significant difference in HbA1c reduction between SGLT2 inhibitor monotherapy, but in combination with metformin SGLTs were more effective at lowering HbA1c than:
- Metformin alone
- Metformin plus SU, or
- Metformin plus DPP4-inhibitor
As SGLT2s work independently of insulin they are associated with a low risk of hypoglycaemia. The AHRQ review found that SGLT2s are associated with greater weight loss than metformin, or metformin plus SU, or metformin plus DPP4.
The most common adverse effects of SGLT2 treatment are genital mycotic infections, e.g. vaginal candidiasis. Rarely, this class has been linked to diabetic ketoacidosis (DKA), so should be used with caution in people at increased risk of DKA, and should be stopped temporarily if the patient is undergoing major surgery or during serious illness.
In one study, an SGLT2 was linked to a two-fold increase in the relative risk of lower limb amputations but the absolute risk remains very low, and the European Medicines Agency has concluded – after considering all the evidence – that the benefits of SGLT2s outweigh risks.
SGLTs inhibitors should not be initiated in patients with an eGFR of
Two of the currently available SGLT2 inhibitors, empagliflozin and canagliflozin, have evidence for reductions in cardiovascular mortality, non-fatal MI and non-fatal stroke, and for significant reductions in progression of renal disease.
SIGN 154 therefore recommends that SGLT2 inhibitors should be considered as add-on therapy to metformin in people with T2D. In individuals with T2D and established CVD, SGLT2 inhibitors with proven cardiovascular benefit (currently empagliflozin and canagliflozin) should be considered.
Glucagon-like peptide-1 (GLP-1) receptor agonists
GLP-1 is a key incretin hormone, secreted from the gut in response to food, prompting secretion of insulin from pancreatic β-cells and inhibiting glucagon secretion. GLP-1 slows gastric emptying, thus slowing absorption of glucose, and increases satiety (feeling full) and reduces appetite. GLP-1 receptor agonists mimic the actions of the naturally occurring hormone. There are five GLP-1s available, as injectable formulations, with varying dosing schedules from one daily to once weekly.
GLP-1s significantly reduce HbA1c as monotherapy, with those with a high baseline HbA1c (>75mmol/mol) experiencing greater reductions. They have also been shown to be effective in combination with metformin, SUs and TZDs.
Severe hypoglycaemia is rare with GLP-1s, occurring only when co-prescribed with an SU. The main adverse effects are gastrointestinal, and related to the mode of action.
GLP-1 treatment is associated with weight loss, typically 1.5 – 3kgs over 6 to 12 months, and is an advantage over treatment with insulin, which is associated with weight gain. Adding a GLP-1 to insulin (as a fixed-ratio combination of liraglutide and insulin degludec [IDegLira]) mitigates weight gain.
Liraglutide has been shown to lead to a reduction in cardiovascular deaths and events, and to reduce the risk of developing nephropathy.
No dose adjustment is needed for people with mild renal impairment, but the use of some GLP-1s should be avoided in people with severe renal impairment. Advice and dose alteration in those with moderate renal impairment varies between individual drugs within the class, and clinicians should refer to the SPC.
SIGN 154 therefore recommends that GLP-1 therapy should be considered in people with a BMI of ≥30kg/m2 in combination with oral glucose lowering agents or insulin or both, as a third or fourth line treatment when adequate glycaemic control has not been achieved. It can also be considered as an alternative to insulin in people for whom treatment with combinations of oral glucose lowering drugs has been inadequate. For people with T2D and established CVD, GLP-1s with proven cardiovascular benefit (currently liraglutide) should be considered.
Insulin
When oral agents no longer provide effective glycaemic control, injectable therapy is required. Insulin provides very effective glucose lowering, but unlike GLP-1s, is associated with weight gain and hypoglycaemia.
Careful clinical judgement should be used to ensure that initiation of insulin therapy is not inappropriately delayed.
When initiating insulin, continuing metformin can help to lower HbA1c and reduce weight gain, without increasing the risk of hypoglycaemia. However, SUs should be discontinued as they increase the risk. The use of other oral therapies should be reviewed on a patient-by-patient basis.
When choosing basal insulin, NPH insulin is as effective as a basal insulin analogue, but the latter is less likely to cause nocturnal or overall hypoglycaemia. The dose of bedtime basal insulin should be titrated against morning (fasting) glucose. If HbA1c does not reach target, consider adding prandial insulin.
Adding in a rapid acting insulin in a premixed biphasic preparation achieves lower HbA1c than a basal insulin analogue alone, but may result in higher total insulin doses than intended, increasing the risk of hypoglycaemia. Care should therefore be taken to optimise the dose and regimen to avoid this risk, and the risk of weight gain.
SIGN 154 therefore recommends that oral metformin should be continued when insulin therapy is initiated to maintain or improve glycaemic control.
Once daily NPH insulin at bedtime should be used when adding insulin to metformin, but basal insulin analogues should be considered according to hypoglycaemia risk.
Aim to optimise the insulin dose and regimen to achieve target glycaemia while minimising the risk of hypoglycaemia and weight gain.
Soluble human insulin or rapid-acting insulin analogues can be used when intensifying insulin regimens to improve or maintain glycaemic control.
SIGN 154. Pharmacological management of glycaemic control in people with type 2 diabetes, November 2017
Related guidelines
View all Guidelines